Why Does an Adhesive Bond?
Why Does an Adhesive Bond?
Adhesives bond as a result of the following three phenomena:
PHASE CHANGES (both a physical and chemical process)
All matter can exist in a number of distinct states, e.g. as a gas, liquid, or solid. The conversion of one state into another is referred to as a phase change. A phase change can occur as the result of either a physical or a chemical process.
An example of a physical process is the transformation of water from a solid state (ice) to liquid to gas (steam). Note that a physical phase change does NOT involve a conversion of material (water remains two hydrogen atoms bonded to one oxygen atom, regardless of the physical state it is in). The energy requirements for physical phase changes are relatively low.
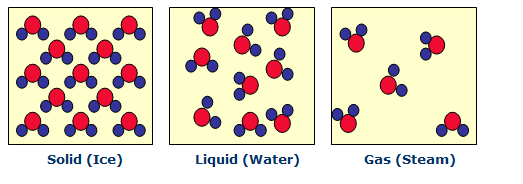
All adhesives must undergo a phase change — from a liquid (at the time the joint is made) to a solid (after cure when the joint has reached final strength).
An example of a chemical process is the combustion of fuel.
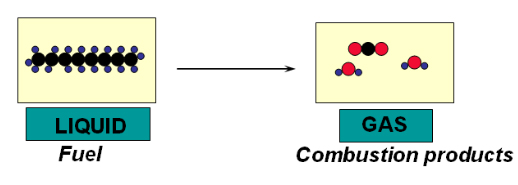
A material conversion takes place (new chemicals are being formed). As chemical bonds are relatively strong, there is a higher energy requirement, compared to a physical phase change, to break them down to create new products.
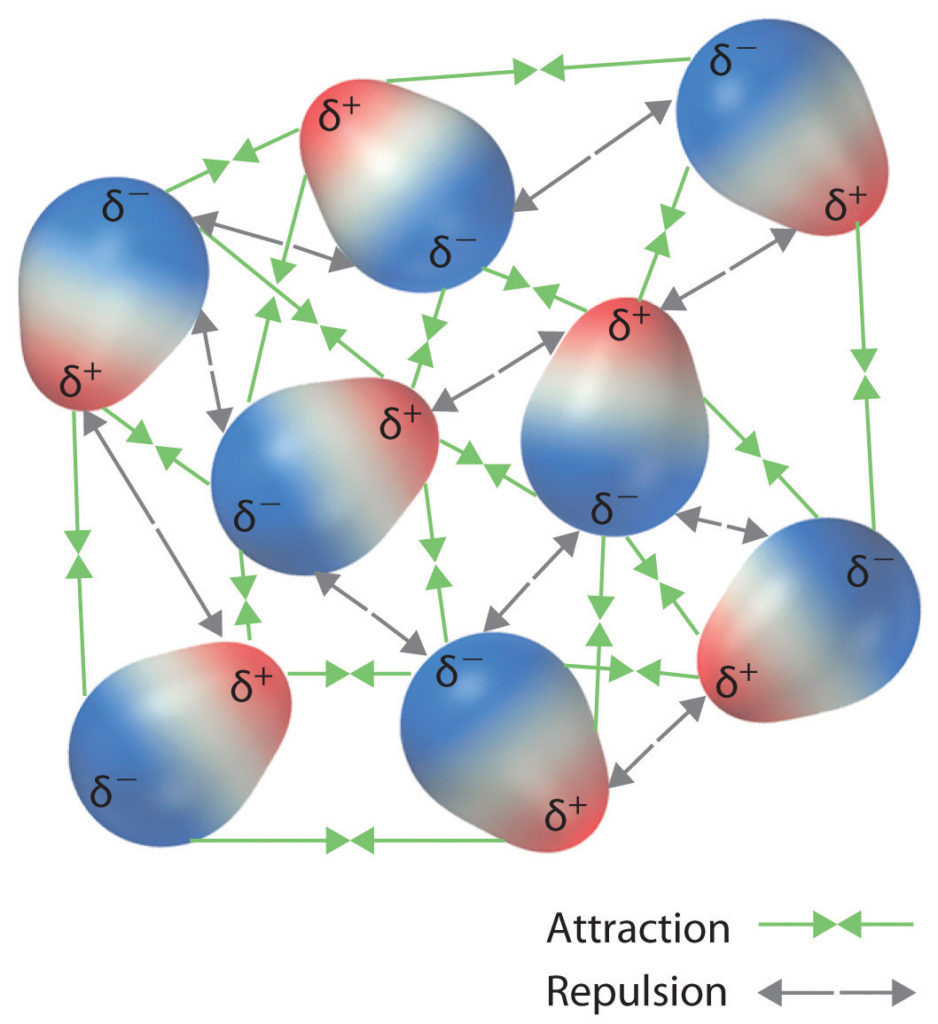
INTERMOLECULAR INTERACTIONS
Chemical bonds — connecting atoms or molecules; electrons are “shared” in the bond, which may be either polar or non-polar.
Physical interactions — Interactions are due to the forces of attraction or repulsion between charges or dipole moments; similar charges repel each other; different charges attract one another.
Micro-mechanical adhesion — At both the molecular and macroscopic levels, micro-mechanical adhesion may occur, but only if there is favorable “spatial configuration” between the molecules and it is a random circumstance(not specifically controlled).
The different types of intermolecular interactions occur only at very small distances, of the order of typical atomic bond lengths. (The range of non-bonding interactions is between 0.3 – 0.5 nm). For interactions to occur, therefore, the two materials must be able to make intimate contact with each other (i.e. they must be able to approach within a nanometer). Note: One nanometer is one-millionth of a millimeter.
Types of Intermolecular Interactions…
| Type of Interaction | Energy (KJ/mol) | Basis of Attraction |
| Bonding | ||
| Ionic | 400-4000 | Cation – Anion |
| Covalent | 150-1100 | Nuclei – Shared Electron Pair |
| Metallic | 75-1000 | Cations – Delocalized Electrons |
| Non-Bonding | ||
| Ion-Dipole | 40-600 | Ion Charge -Dipole Charge |
| Hydrogen Bonding | 10-40 | Polar Bond to Hydrogen – Dipole Charge |
| Dipole – Dipole | 5-25 | Dipole Charges |
| Ion – Induced Dipole | 3-15 | Ion Charge – Polarizable Electrons |
| Dipole – Induced Dipole | 2-10 | Dipole Charge – Polarizable Electrons |
| Dispersion Forces | 0.1-40 | Interaction Between Polarizable Electrons |
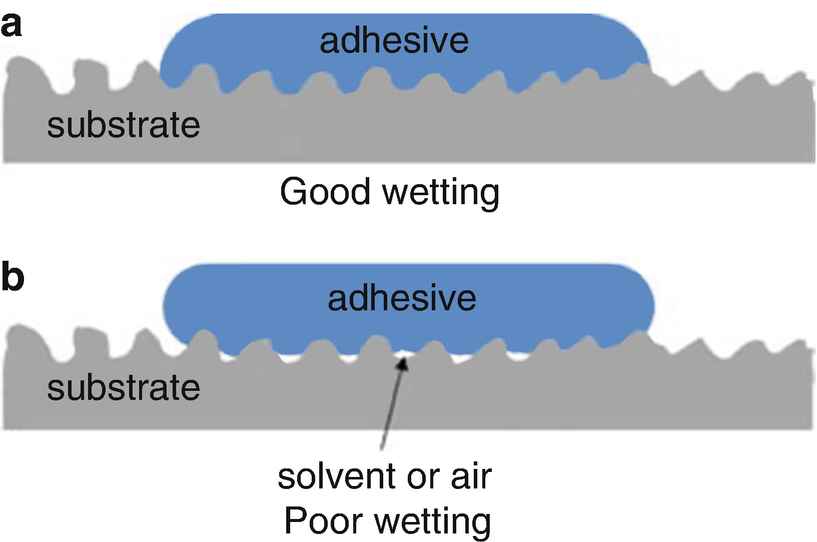
WETTING
A prerequisite for forming the adhesive boundary layer is good wetting of the substrate surface by the liquid adhesive. The degree of wetting, which among other things is determined by the surface tension of the adhesive and substrate, is hence a criterion for the quality of the adhesion. The approaching of atoms is however only a prerequisite for the formation of adhesive forces. The determining factor for the actual adhesion is the accessibility of and number of physically or chemically active structures on the substrate surface and in the adhesive.
The wetting of the substrate surface by the liquid adhesive is necessary for adhesion, but this alone is not sufficient. Good wetting alone does not necessarily guarantee the desired good long-term adhesion of the adhesive to the surface.
Consider, for example, high-grade steel: Although this has a high surface tension it can be easily wetted. However, due to its passive character (poor bonding properties) there is only relatively poor adhesion of adhesive to the surface.
If the substrate surface is incompatible with an adhesive – for example because the liquid adhesive does not adequately wet the surface or because the adhesive bonds are too weak – then the surface can be coated with a suitable adhesion promoter. These adhesion promoters function via different bifunctional chemical groups. Some of the groups are adapted to the chemistry of the substrate surface, whilst others are adapted to the adhesive. The most common adhesion promoters bond chemically to both substrates. Surface treatment methods give other options for enhancing the wetting of the substrate surface.
Adhesion makes an important contribution to the strength of a bonded joint. Users can significantly affect the adhesion by:
Ensuring the surfaces of the substrates are clean and, if necessary, pretreating the substrate surfaces,
Selecting an adhesive, and if necessary an adhesion promoter/primer, that is suitable for the chemistry of the substrate surfaces.
However, an immediate conclusion cannot be drawn relating the (microscopic level) adhesion to the macroscopic joint strength (and vice versa). The macroscopic cohesive properties of an adhesive (e.g. cohesion strength, elastic behavior) are largely determined by the choice of the base adhesive and the adhesive formulation, and can be little influenced by users.

